Improving and Removing Bling Spots in Medical Device Testing
In the realm of medical devices, precision isn’t optional—it’s imperative. Every circuit, sensor, and embedded system must perform flawlessly, because lives depend on it.
Aligning ISO 13485, which is key for medical device quality systems, with IEEE 1149.1 (JTAG) is essential. IEEE 1149.1 is the standard for testing PCB boards using the boundary-scan method. This alignment is not just a good practice; it is a strategic necessity.
ISO 13485: Engineering Confidence Through Process Discipline
ISO 13485 defines the framework for quality management in medical device development. It mandates rigorous documentation, risk management, and tracing faults across the product lifecycle. For engineering leaders, it provides the structure to scale innovation without compromising compliance.
Key pillars include:
- Lifecycle-spanning risk management
- Design controls and validation
- Attributing faults and corrective/preventive actions (CAPA)
But while ISO 13485 ensures process integrity, it doesn’t address how to embed testability into the hardware itself. That’s where you need IEEE 1149.1 to maintain a rigorous testing process.
IEEE 1149.1: Built-In Visibility for Embedded Systems
IEEE 1149.1, also known as JTAG, allows easy access to internal circuits. This helps engineers check connections, program devices in the system, and run diagnostics in the field. You can do all this without using physical probes. For complex embedded systems, it’s a foundational tool for ensuring functional integrity from prototype to production.
Benefits include:
- Structural and functional test coverage without intrusive fixtures
- In-system programming and real-time debugging
- Increased speed of root-cause analyzing in the field.
In high-reliability sectors like aerospace and defense, JTAG is standard. For medical devices, it’s a powerful yet under used asset.
Where Standards Converge: A Closed-Loop Advantage
Integrating IEEE 1149.1 into ISO 13485-compliant workflows creates a virtuous cycle of quality and efficiency. Here’s how:
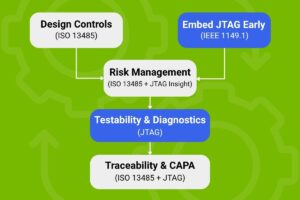
This diagram illustrates the integration of ISO 13485 quality management with IEEE 1149.1 boundary-scan testing in medical device development.
Design for Testability (DfT) Meets Design Controls
Embedding JTAG early in the design phase enhances verification and supports ISO-mandated documentation. Test results become part of the design history file (DHF), streamlining audits and fast tracking design reviews.
Risk Management with Real-Time Insight
JTAG enables early fault detection and supports ISO 14971-compliant risk mitigation. We can identify and resolve latent hardware issues before they impact patient safety or regulatory standing.
Removal of Blind Spots and CAPA Integration
When failures occur, JTAG provides actionable diagnostics that feed directly into CAPA workflows. This shortens feedback loops and strengthens continuous improvement efforts.
Practical Guidance for Engineering Leaders
To implement this integration:
- Embed early: Incorporate JTAG from the first prototype. Retrofitting is costly and limits test coverage.
- Educate cross-functional teams: Ensure design, test, and quality engineers understand how JTAG complements ISO 13485.
- Automate strategically: Use tools like ScanWorks to automate boundary-scan diagnostics and integrate with your QMS.
- Document rigorously: Align test data with ISO documentation to simplify regulatory submissions.
Why It Matters
For engineering directors navigating the dual pressures of innovation and compliance, this convergence offers a path forward. Not about adding complexity—about engineering smarter.
By using IEEE 1149.1 for diagnostics and ISO 13485 for process rules, teams can improve device safety. This approach also helps in making devices more reliable. Additionally, it allows teams to work faster.
This is where ScanWorks excels. Trusted by leaders in medical technology, ScanWorks empowers engineering teams to validate, debug, and document with confidence—without compromising speed or compliance.
In medical devices, quality is not just a deliverable. It’s a promise to patients, to regulators, and to the future of healthcare. When engineering directors align diagnostic precision with process integrity, they do more than meet standards They define them. ScanWorks helps make that possible, turning compliance into a catalyst for innovation.

